Emergency Department Intervention Reduces Adult Suicide Risk
• Research Highlight
Suicide is a leading cause of death in the United States, but there are opportunities to intervene and save lives. Many people who die by suicide visit an emergency room in the weeks or months before, making them critical places to reach people at risk. Addressing suicide risk in these fast-paced, urgent care settings is challenging, but research funded by the National Institute of Mental Health showed that it is possible. The study, known as the Emergency Department Safety Assessment and Follow-Up Evaluation 2 (ED-SAFE 2) , significantly reduced suicidal behaviors among people at risk of suicide.
What is the ED-SAFE suicide risk intervention?
ED-SAFE is a randomized clinical trial designed to improve suicide risk screening and detection in emergency department settings. The trial differs from similar studies because routine clinical staff—rather than researchers—deliver the suicide risk intervention. This approach increases the likelihood that the suicide prevention strategies can be reliably implemented and sustained in the real world. ED-SAFE is also the largest practical clinical trial of suicide-related best practices in emergency departments.
The original ED-SAFE study showed that universal suicide risk screening and a multicomponent intervention for adults at risk of suicide could be implemented in emergency departments. The intervention consisted of safety planning to help patients identify early warning signs and access resources and support and was followed by telephone outreach after leaving care. It doubled the number of patients identified as at risk for suicide and reduced subsequent suicidal behaviors by 20%.
What did researchers do in the current study?
The current study was the next step in this research effort. Edwin Boudreaux, Ph.D. , at the University of Massachusetts Chan Medical School, led a multidisciplinary team in examining whether a package of suicide prevention efforts focused on improving department-wide clinic workflow could reduce suicide-related behaviors. Having established that the suicide risk intervention was feasible and effective, the researchers wanted to see if it could be improved further using a science-based approach focused on quality improvement.
The multisite study was conducted at eight demographically diverse emergency departments across the United States. Participants were adults seen for emergency care between January 2014 and April 2018.
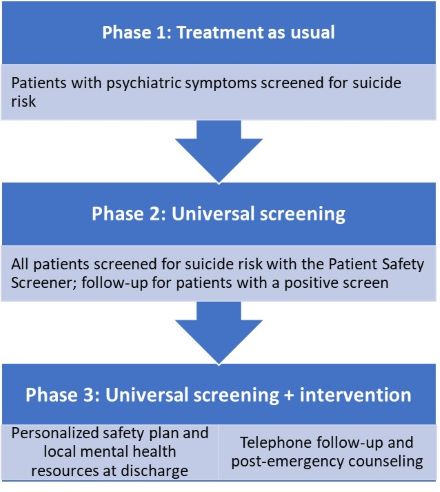
Quality improvement experts and suicide prevention specialists trained staff at the sites to evaluate their current suicide-related workflow, identify gaps and opportunities for growth, and design solutions to gradually improve care. Clinical staff implemented these efforts in a staggered fashion over three 12-week phases.
- Baseline phase: Continuing the enhanced suicide prevention efforts adopted in the original ED-SAFE study, including universal suicide risk screening
- Implementation phase: Introducing improvements to suicide-related care, including collaborative safety planning between clinicians and patients to help manage future suicidal crises
- Maintenance phase: Sustaining gains from the implementation phase, regularly evaluating workflow, and making ongoing enhancements to care
During each month of the study, the researchers randomly selected 25 patients at each site from the pool of all patients who screened positive on a validated suicide risk screener. Although all patients found to be at risk for suicide received the ED-SAFE suicide risk intervention, the researchers reviewed the medical records of only the selected patients to see whether they received care for suicidal ideation or a suicide attempt or died by suicide within 6 months of their initial emergency visit.
Did ED-SAFE 2 improve suicide-related care?
In analyzing data from almost 7,000 medical records, the researchers observed a significant change in suicide risk over the study phases. The likelihood of suicidal ideation, attempt, or behavior in the 6 months after an emergency visit was significantly lower during the maintenance phase compared to the baseline phase, reflecting a 30% decrease. These results showed that implementing best practices for suicide care, including universal screening and collaborative safety planning, and evaluating them on a continuous basis effectively improved suicide-related outcomes following emergency discharge.
The current researchers improved on the reduction in suicide risk observed in the original ED-SAFE study achieved using comprehensive suicide prevention efforts. They did so by adding quality improvement methods to help emergency department staff regularly monitor and adjust their clinical practices over time. These findings highlight the lifesaving benefits of incorporating brief suicide interventions into routine emergency care. Importantly, clinical staff in real-world emergency departments could feasibly and consistently provide this care. If applied more widely, these suicide prevention strategies could enhance clinical care, decrease suicide risk, and save lives.
If you or someone you know is struggling or having thoughts of suicide, call or text the 988 Suicide and Crisis Lifeline at 988 or chat at 988lifeline.org. In life-threatening situations, call 911.
Reference
Boudreaux, E. D., Larkin, C., Sefair, A. V., Ma, Y., Li, Y. F., Ibrahim, A. F., Zeger, W., Brown, G. K., Pelletier, L., Miller, I. & ED-SAFE 2 investigators. (2023). Effect of an emergency department process improvement package on suicide prevention: The ED-SAFE 2 cluster randomized clinical trial. JAMA Psychiatry, 80(7), 665−674. https://doi.org/10.1001/jamapsychiatry.2023.1304
